General Introduction:-
- Cis diols is formed by KMnO4 & OSO4.
- Trans diols using peroxide and metal carbohydrate etc.
Reaction By Using KMnO4:-
Mechanism :-
Upjohn Oxidation:- It allow the syn selective preparation 1,2-diols from alkenes by the use of osmium tetraoxide as a catalyst and a stochiometric amount of an oxidant such as NMO (N-methyl morpholine-N-Oxidation)
Reaction:-
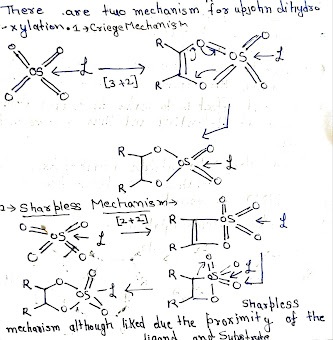
There are two mechanism for upjohn dihydroxylations:-
- Criege Mechanism
- Sharpless Mechanism
Important Points :-
- NMO used as a reoxidant for osmium tetraoxide.
- It will dehydroxylate all the alkene (but only alkenes)
- Electron withdrawing group on the alkenes slow the reaction but do not stop it.
- Bulk of osmium complex results in dihydroxylation from least hindered face.
- Criege showed that the amine ligands increase rate o0f reaction.
- Presumbly an electron rich complex is formed increasing the reactivity of the oxygen atom.
- A second effect is to make the oxygen atoms better at H- bonding and thus reverse slecturty.
- Green Context:- Use a catalytic amount of toxic and volatile OSO4 due to the presence of NMO as reoxidant.
Next Topics:- Woodward cishydroxylation










thank you for your feedback