Introduction:-
The Raman effect was named after one of its discoverers, the Indian scientist C. V. Raman, who observed the effect in organic liquids in 1928 together with K. S. Krishnan, and independently by Grigory Landsberg and Leonid Mandelstam in inorganic crystals.
Raman won the Nobel Prize in Physics in 1930 for this discovery.
The first observation of Raman spectra in gases was in 1929 by Franco Rasetti.
Quantum Theory of Raman Effect :-
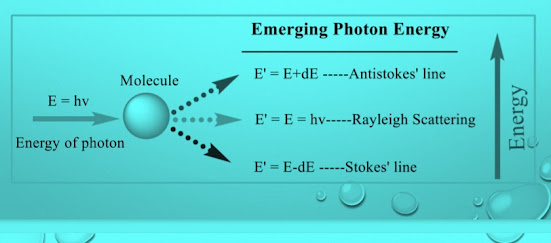
Raman Scattering could be understood in terms of the quantum theory of radiations.Photons can be imagined to undergo collisions with molecules.
Radiation scattered with a frequency lower than that of the incident beam is referred to as Stokes' radiation, while that at higher frequency is called anti-Stokes' radiation.
The Raman effect is the inelastic scattering of photons by molecules or solids, resulting in emitted photons with different energies (and thus wavelengths) than the incident photons.
The quantum theory of the Raman effect explains the phenomenon by considering the interaction between incident photons and vibrational modes of molecules or solids. This theory explains the selection rules and probability amplitudes of these interactions and predicts the intensity and polarization of scattered light. This theory also plays an important role in interpreting Raman spectroscopy, a widely used technique for studying vibrational modes of molecules and solids.